Electrophotocatalysis
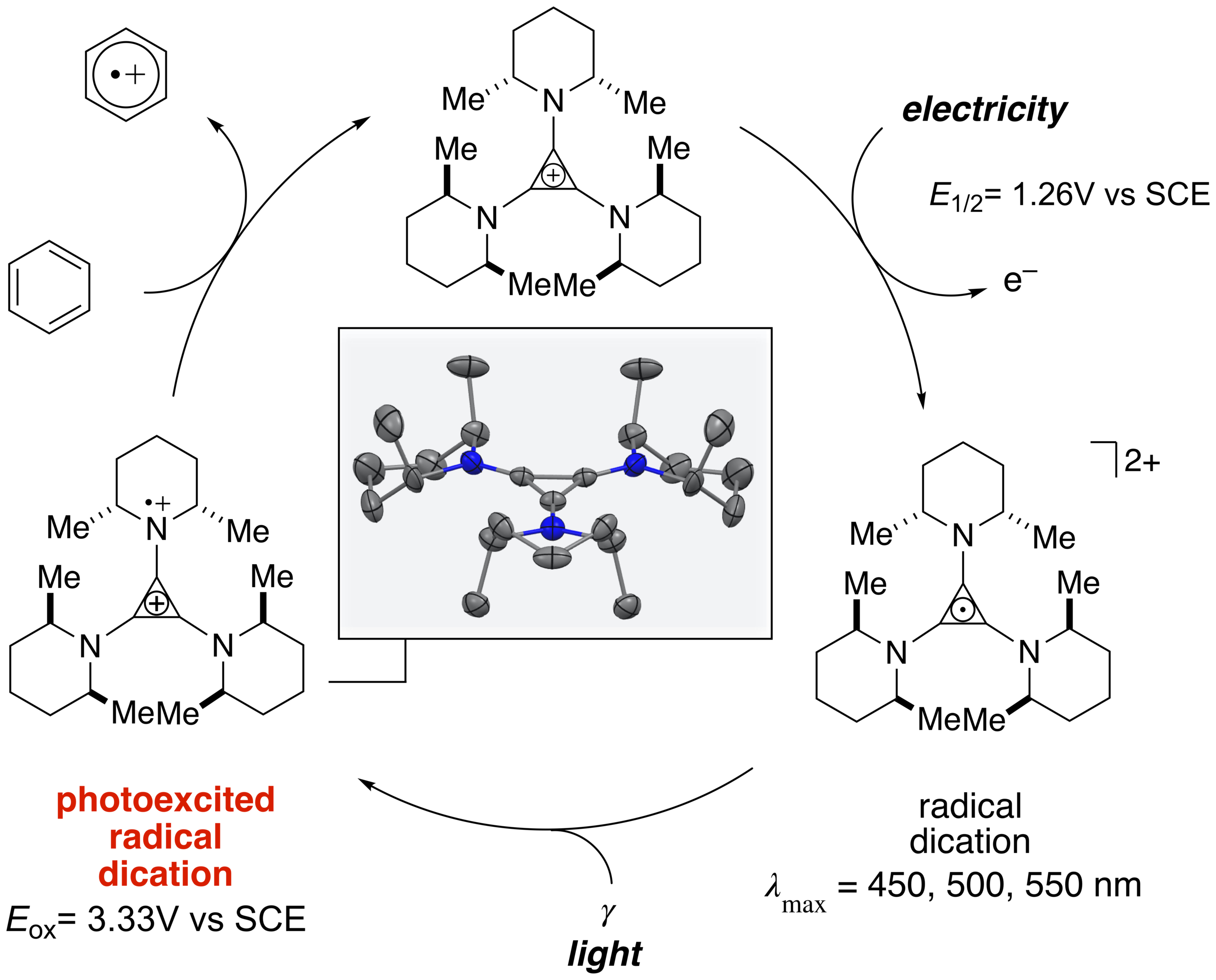
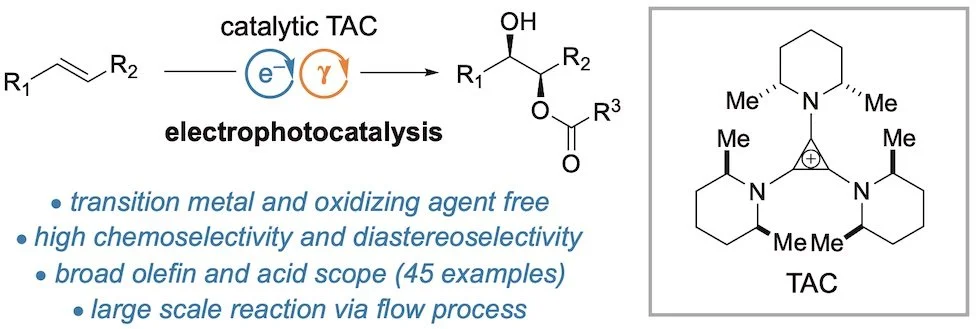
Both photocatalysis and electrocatalysis have had a transformational effect on organic synthetic chemistry over the last decade. Given this impact, a natural question to ask is how might the energy of light and electricity be combined within a single catalytic process? Until recently, very few examples of such “electrophotocatalysts” had been described. We have shown that trisaminocyclopropenium (TAC) ions can serve as electrophotocatalysts for potent oxidation reactions (Angew. Chem. Int. Ed. 2019, 58, 13318). This catalysis is based on the mild electrochemical oxidation of the TAC to form a stable radical dication, which can then be photoexcited with visible light to generate an excited state with an estimated reduction potential of well over 3 V. We have shown that this level of oxidizing power is enough to functionalize benzene and halobenzenes cleanly and selectively. We have also shown that ethers can be electrophotocatalytically functionalized with high regioselectivity (J. Am. Chem. Soc. 2020, 142, 1698). A key aspect of the TAC catalyst is the cis-dimethylpiperidine units, which protect the ion from photodegradation due to its all-cis conformation. For a perspective article on Electrophotocatalysis, see J. Am. Chem. Soc. 2022, 144, 12567).
Amination of C–H Bonds
TAC electrophotocatalysis can be used to achieve a Ritter-type amination of benzylic C–H bonds, using simple acetonitrile as the nitrogen source (J. Am. Chem. Soc. 2021, 143, 8597). With modified conditions, a remarkable vicinal C–H diamination occurs, leading to the production of 3,4-dihydroimidazoles from the saturated precursors (Science 2021, 371, 620).
Aryl Olefin Functionalization
Electrophotocatalysis can also lead to the difunctionalization of aryl olefins, even though such substrates can be sensitive to oxidizing and acidic conditions. For example, we have shown the acetoxyhydroxylation (J. Am. Chem. Soc. 2021, 143, 7247) and the regiodivergent aminooxygenation (J. Am. Chem. Soc. 2022, 144, ASAP) of aryl olefins.
Reductive Electrophotocatalysis
In collaboration with the group of Prof. Song Lin, we have also demonstrated electrophotocatalysis is effective for realizing very strongly reducing conditions as well (J. Am. Chem. Soc. 2020, 142, 2087). Specifically, we showed that the mild cathodic reduction of dicyanoanthracene generates the corresponding radical anion, which when photoexcited is nearly as reducing as lithium metal. The secret sauce behind both the oxidative and reductive electrophotocatalytic strategies is the use of open-shell, doublet photocatalytic intemediates to achieve extreme redox potentials with only mild inputs of light and electrical energy.
Electrophotocatalytic SnAr
In addition to the open-shell work, we have also found that potent photooxidants like DDQ can be engaged as useful electrophotocatalysts as well (Angew. Chem. Int. Ed. 2020, 59, 658). For example, we showed that classically unactivated aryl fluorides can undergo nucleophilic substitution with pyrazoles, alcohols, or other nucleophiles at room temperature and without strong base (in fact in the presence of acetic acid). The peculiarities of the process enabled us to achieve selective SnAr reactions of chlorofluorobenzene in the presence of either an electron-deficient arene (2,4-dinitrofluorobenzene) or an electron-rich arene (4-methoxyfluorobenzene).