Catalytic Carbonyl-Olefin Metathesis
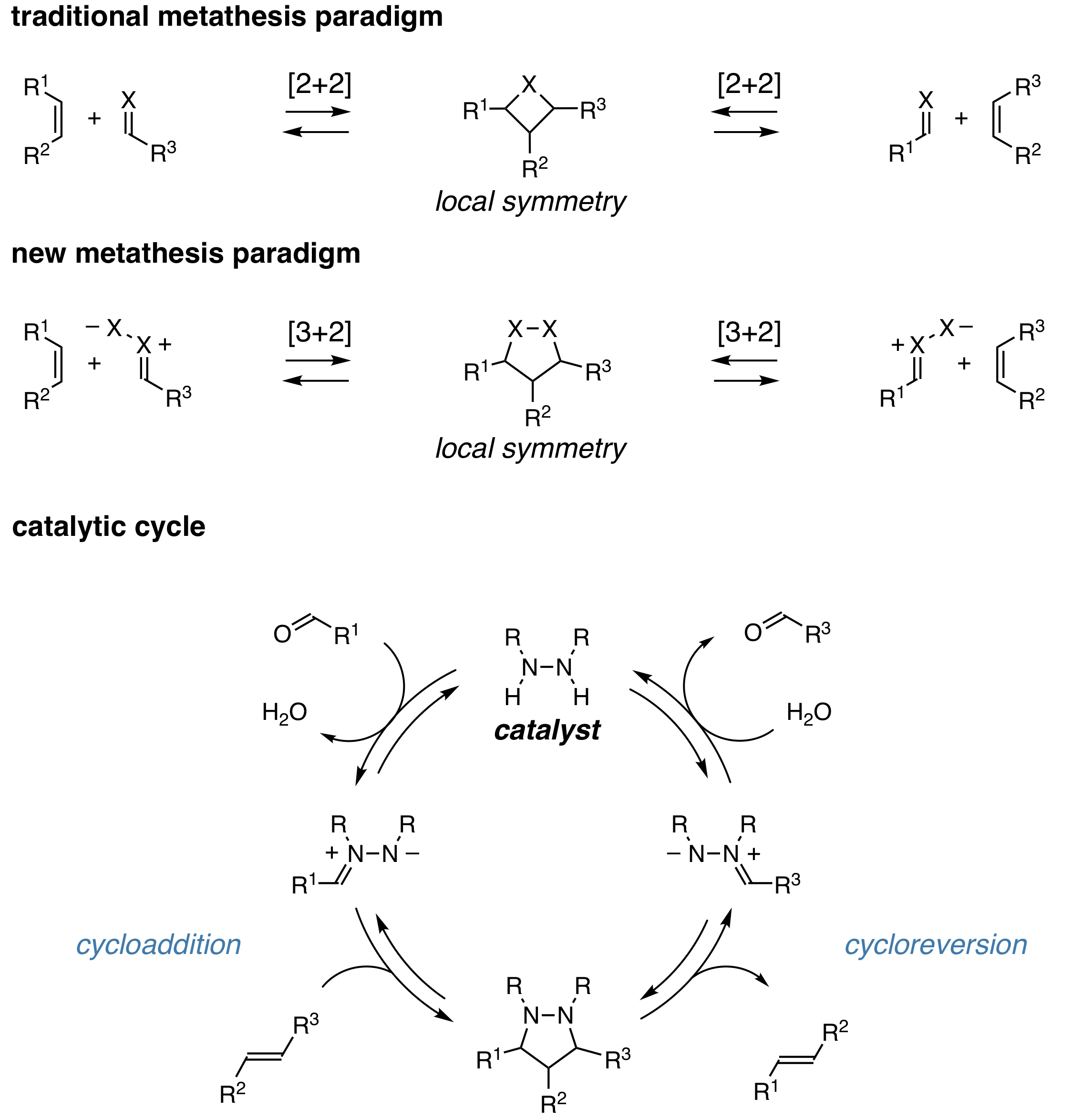
Double bond metathesis reactions, especially catalytic olefin metathesis and the Wittig reaction, have had a large impact on the field of chemical synthesis. Despite an equally high synthetic potential, carbonyl-olefin metathesis has seen relatively little progress until very recently, particularly in the context of catalysis. Our group developed the first catalytic strategy for carbonyl-olefin metathesis by using a paradigm of [3+2] cycloaddition/cycloreversion rather than the ubiquitous [2+2] manifold employed by existing strategies. The catalytic design centered on the use of simple symmetric hydrazines as catalysts, which effect metathesis through a sequence of reversible condensation and cycloaddition steps. For a review on this catalysis, see SynLett 2019, 30, 1954. For a general review on carbonyl-olefin metathesis we published with Corinna Schindler, see Chem. Rev. 2021, 121, 9359.
ROCOM of Small-Ring Olefins
We first reported the implementation of this strategy with the ring-opening carbon-olefin metathesis (ROCOM) of cyclopropenes using a [2.2.1]-bicyclic hydrazine as the catalyst (J. Am. Chem. Soc. 2012, 134, 18581; Chem. Sci. 2014, 5, 471). More recently, we extended this concept to cyclobutenes, which delivers formal Claisen rearrangement products (ACS Catal. 2022, 12, 4813).
ROCOM of Norbornenes
With the help of computational design, we have identified a second-generation catalyst that allows for the ROCOM of norbornenes (Chem. Sci. 2020, 11, 7884). Further catalyst optimizations should enable the expansion to other, less-strained olefins.
Ring-Closing Carbonyl-Olefin Metathesis
We have also shown that hydrazine catalysis can be used for ring-closing carbonyl-olefin metathesis (RCCOM), for example to generate 2H-chromenes (ACS Catal. 2019, 9, 9259), dihydroisoquinolines (Org. Lett. 2020, 22, 6026), or polycyclic heteroaromatics (Chem. Sci. 2022, 13, 2418). Particularly for the latter work, we showed that hydrazine catalysis is compatible with strongly Lewis basic functionality, which strongly inhibits acid-catalyzed approaches.
A New System for Olefin Metathesis and ROMP
The same logic that enables carbonyl-olefin metathesis can be applied to achieve olefin metathesis without the need for metals. We demonstrated this idea with the hydrazonium-mediated ring-opening metathesis polymerization (ROMP) of cyclopropenes in a collaborative paper with Brett Fors (Angew. Chem. Int. Ed. 2022, 61, Early view).